Abstract
Acute Myeloid Leukaemia (AML) is thought to occur due to stepwise accumulation of mutations in haematopoietic stem and progenitor cells (HSPCs) or early myeloid progenitor cells (EMPs) to either block differentiation or increase proliferation. In around 10% of sporadic AML cases and several known germline predisposition kindreds the myeloid transcription factor CEBPA is mutated. 50% of cases possess biallelic mutations in both C and N terminae (dm) and 50% monoallelic mutation (sm). Using TALENs we have created both C and N terminal mutants in zebrafish to study their cooperating effects in the development of AML.
Our first observation was of striking defects in mature myelocytes and monocytes in all double mutants (cebpamut/mut) as assessed by Sudan black staining at 5 days post fertilisation (dpf) and apoeb whole mount in situ hybridisation (WISH) at 3dpf. In situ hybridisation revealed defects in myelopoeisis in all cebpamut/mut fry as early as 28hpf, with markedly decreased expression of coronin and l-plastin.
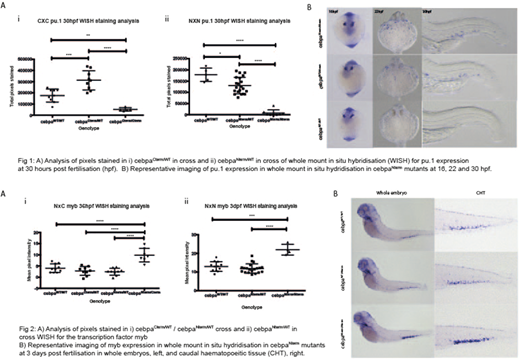
We then interrogated the model further to assess when and where the differentiation block was occurring. We utilised the transcription factor pu.1 both as an in vivo transgenic marker Tg(pu.1:GFP) and WISH probe at earlier time points. In primitive haematopoiesis (until 22hpf) pu.1 expression showed no defects in dm mutants. However, pu.1 expressing cells were markedly reduced in dm mutants in establishment of myelopoeisis in the caudal haematopoietic tissue (CHT), fetal liver equivalent. Following formation of the CHT at around 30hpf, differing patterns of pu.1 expression were seen in sm mutants, with cebpaWT/Cterm having significantly increased staining, which was reduced in cebpaWT/Nterm (see figure 1).
HSPC numbers in cebpamut/mut were seen to be normal by WISH for gata2b and runx1 and flow cytometry in Tg(CD41:GFP) fry during early haematopoeisis (2-5dpf). However, myb expression was markedly increased at 36hpf and 3 days but only in cebpaCterm/Nterm and cebpaNterm/Nterm dm mutants (see figure 2). This suggests an accumulation of early progenitors with myeloid potential but not commitment, as implied by absence of pu.1 expression but normal CD41lo numbers and other stem cell marker expression.
Absence of MCherry expression in the transgenic LysC:MCherry, which highlights mature myeloid cells in vivo, was sufficiently reliable to genotype all cebpamut/mut fry and observe them for impaired survival compared to wild-type and heterozygous siblings and for the development of leukaemia. Similarly to the increase in myb staining, leukaemic transformation was only observed in dm mutants with at least one N terminal mutation, as assessed by development of anaemia and florid Tg(CD41:GFP) expression. Flow cytometry in juvenile fish (4-6weeks) also identifies a developing pre-leukaemic phenotype with moderate yet significant expansion of HSPCs and continuing absence LysC expression in cebpaNterm/Cterm and cebpaNTerm/NTerm. However, survival is poor in all dm fish, likely due to the metabolic role of cebpa and vulnerability to infection secondary to the severe myelomonocytic defect.
Our results show that absence of WT cebpa has dramatic effects myelopoiesis from early stages of differentiation in definitive haematopoiesis. In addition, accumulation of myb expressing progenitors occurs as they arise from the aorta in dm mutants, identifying this as a sub-population of HSPC vulnerable to further leukaemogenic hits. Ongoing work will define the mechanism of these effects in sm and dm mutants and include comparative expression profiling in myb and pu.1 positive cells.
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal